|
II-VI compounds: generally, compounds formed by elements from II & VI groups of the Periodic table. Here we mean only binary (ZnSe, ZnS, ZnTe, CdSe, CdS, CdTe) and ternary (like, e. g., CdZnTe or CdSSe) zinc and cadmium chalcogenides that are wide-gap semiconductors. HPVB: High-Pressure Vertical Bridgman method. Represents modified Bridgman growth, carried out under high (usually 10-100 atm) inert gas pressure. Developed and first time applied for II-VI compound crystal growth in our lab back in late sixties [1]. So far it is the most widespread method for cadmium and zinc chalcogenide growth, though it has some drawbacks. HPVZM: High-Pressure
Vertical Zone Melting. The most advanced solution for cadmium and zinc
chalcogenide growth from the melt. Invented in our lab back in mid-eighties
[2-4]. Recently modified for growth of ternary II-VI compounds [5].
|
|||
|
The II-VI compounds have particular physical-chemical properties that present a challenge for the crystal growth. Zinc and cadmium chalcogenides are refractory, chemically corrosive (in liquid and vapor state) materials with high pressures of their own vapors at temperatures exceeding their respective melting points. While evaporating, II-VI compounds dissociate to the components; the metals evaporate in atomic form and chalcogens – in molecular one (in general case as B2, except the tellurium vapors at low temperatures). The typical crucible material – graphite – is partially permeable for vapors of II-VI compounds and their components. As a result, the composition of the melt shifts to an excess of one of the components due to a difference of the vapors diffusion coefficients in an external medium, which is an inert gas (most typically - argon). In the general case of II-VI compounds and HPVB growth conditions:
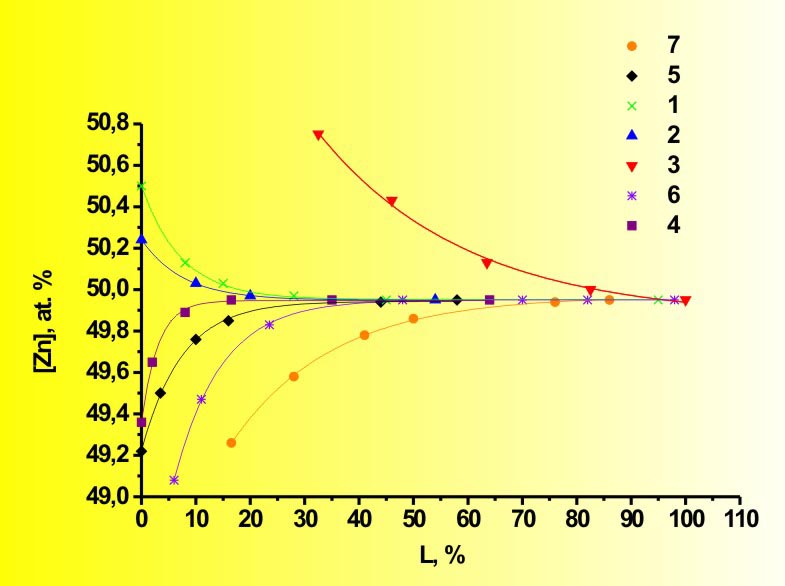
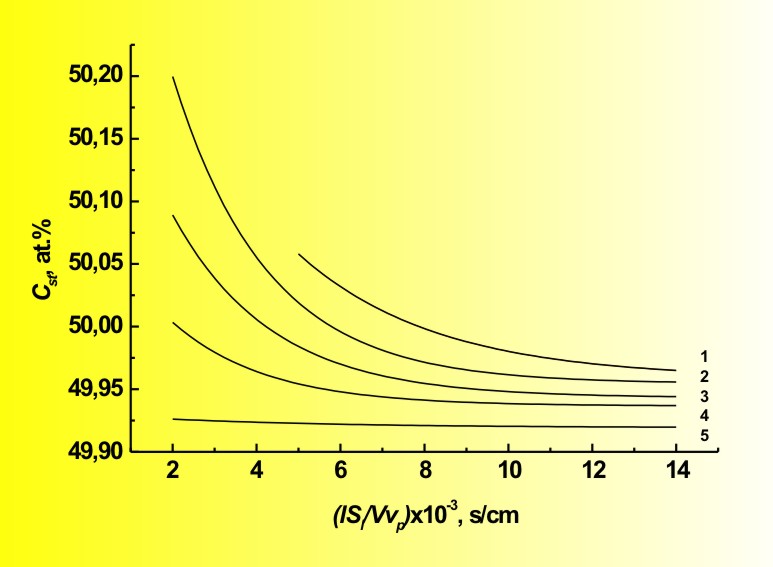
That really means impossibility of preparing stoichiometric crystals by the HPVB. Moreover, in general case the II-VI compound has so-called "quasiequilibrium" composition, which differs from stoichiometric one. It is “quasiequilibrium”, because the theory allows only asymptotic approach to it. The one exception is ZnS - for this compound the "quasiequilibrium" composition coincides with the stoichiometric. The HPVZM solves that problem providing means for the
main composition control during the crystal growth.
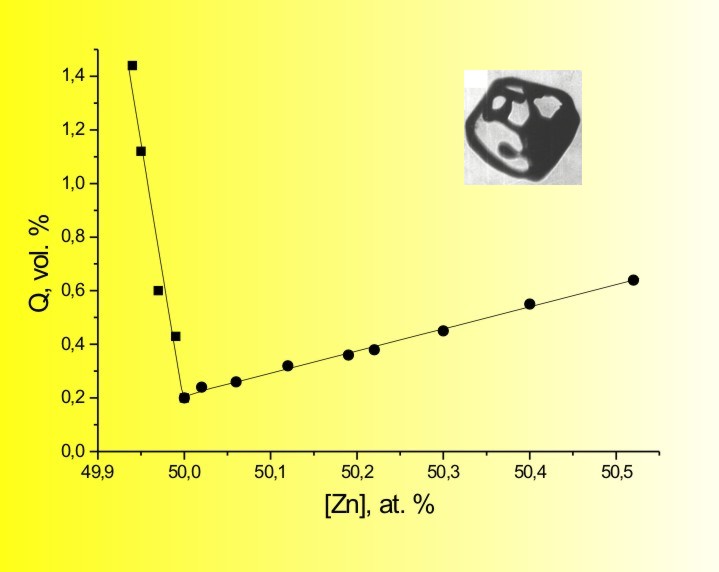
Of course, these so-called "quasistationary" compositions could be also approached only asymptotically, but, nevertheless, the HPVZM allows obtaining any II-VI crystals with practically stoichiometric composition on about 70 % of the ingot length (while the HPVB permits that only for ZnS). The theory is valid for all binary and ternary II-VI compounds, though in case of the tellurides the discrepancies with analysis are perceptibly higher, because the calculations do not consider existence of some Ten molecules with n > 2. These peculiarities of composition stipulate another problem
of the II-VI compound crystal growth from the melt - "bubbles". The "bubbles"
are in fact voids in shape of formed or semi-formed negative crystals (in
case of selenides and sulfides) or tellurium inclusions (in case of tellurides).
They originate in the melt state as bubbles filled with the vapors of free
components (liquid and vapor in case of Te) and then are captured by the
growing crystal.
In simply words any II-VI compound crystal, grown from
the melt, has some bubbles due to the permanent melt dissociation. The
closer the composition is to the stoichiometry, the lower the bubble content
is (the HPVZM again has an advantage here).
|
|||
|
|
|||
|
[1] M. P. Kulakov, A. V. Fadeev. Device for crystal
growth by vertical direct crystallization of melt. Pat. USSR N 774286.
|